TRIALS
REVERS: Study with postbiotics in people with AMD
STATUS:
En reclutament
DESCRIPTION
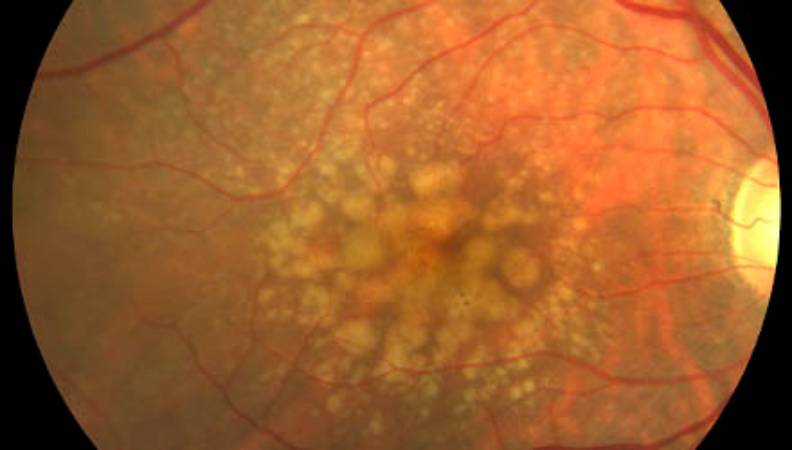
Objectives
Study to evaluate the efficacy and safety of postbiotic supplementation in:
- The prevention of AMD progression and its impact on the worsening of functional and anatomical assessment criteria in intermediate age-related macular degeneration (AMD).
- Slowing the progression rate of geographic atrophy (GA) in patients with late non-exudative AMD or macular dystrophies.
- Improving the efficacy of anti-angiogenic therapy in patients with choroidal neovascularization and insufficient treatment response.
Depending on the treatment group, participants will be randomly assigned either a nutritional supplement approved for the prevention of intermediate AMD progression (AREDS) or the same supplement enriched with postbiotics.
Population
Patients over 50 years of age with intermediate AMD, geographic atrophy, and choroidal neovascularization undergoing anti-angiogenic treatment with a poor response to therapy.
Duration
L’estudi tindrà una durada de dos anys amb una possible extensió a tres anys
Associated Pathologies
No results found.
If you found this interesting, feel free to share it here:
Última modificació: 01/09/2025

