TRIALS
IONIS: evaluation of the safety and efficacy of ISIS 696844 in patients with geographic atrophy secondary to AMD
STATUS:
Pausat
DESCRIPTION
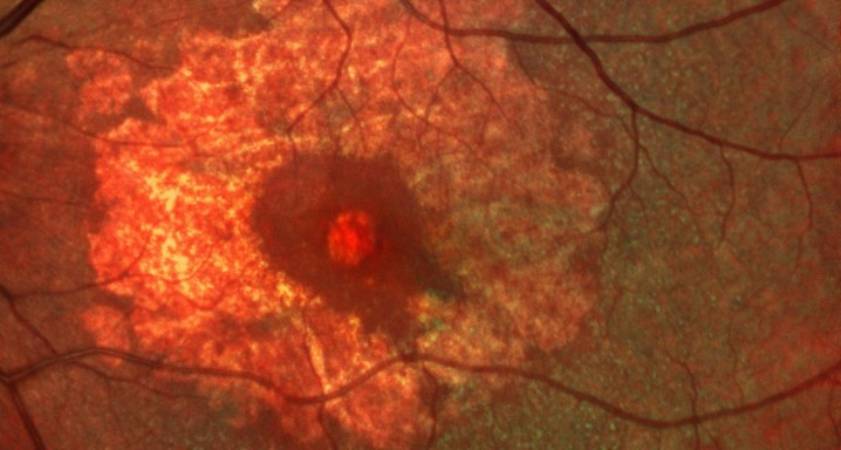
This is a Phase II, randomized, placebo-controlled, double-masked study to evaluate the safety and efficacy of different doses of IONIS-FB-LRX, an antisense oligonucleotide inhibitor of Complement Factor B, in patients with geographic atrophy secondary to age-related macular degeneration (AMD).
Objectives
- Evaluate the effect of ISIS 696844 on the rate of change in the area of atrophy measured by autofluorescence imaging.
- Evaluate the effect of ISIS 696844 on plasma levels of Complement Factor B and serum AH50 activity in patients with AMD.
- Evaluate the effect of ISIS 696844 on the slow decline of visual acuity under low-light conditions.
Drug
FARMAC:
The injection of the compound ISIS 696844 (40, 70, 100 mg) or placebo will be administered subcutaneously at weeks 1, 3, and 5 of the study, and then every 4 weeks until week 45.
Population
INCLUSION CRITERIA:
- Men or women aged 50 years or older
- Vaccination against meningitis and pneumonia
- Diagnosis of unilateral or bilateral AMD
- Total atrophic area due to AMD between 1.9 and 17 mm²
Duration
La durada total de l’estudi serà d’aproximadament 18 mesos.
Associated Pathologies
No results found.
If you found this interesting, feel free to share it here:
Última modificació: 01/09/2025

