Rare diseases (also called orphan or uncommon diseases) are defined as those affecting 1 or fewer in every 2,000 people. There are around 10,000 types of conditions considered rare, many of which have no treatment. Research is the only strategy to develop therapies for these individuals.
Hereditary retinal dystrophies (HRDs) are a heterogeneous group of rare eye diseases caused by mutations in a single gene (monogenic or Mendelian). More than 300 genes responsible for HRDs have been identified.
Below you will find more information on each of them:
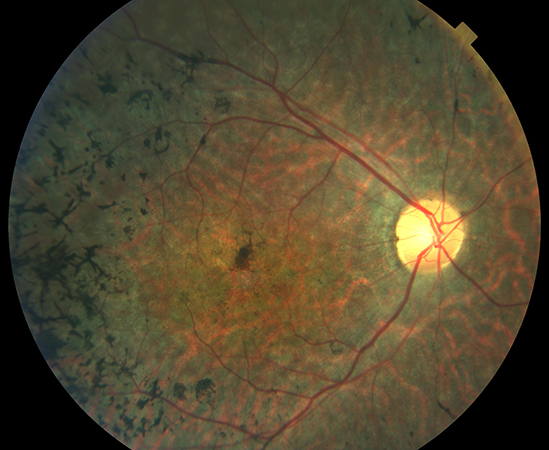
Stargardt disease (OMIM 248200) is one of the most common macular hereditary retinal dystrophies. Most cases are inherited in an autosomal recessive pattern. The visual decline caused by this disease typically affects young people, especially teenagers and young adults under 20 years of age (1:10,000). Stargardt disease and fundus flavimaculatus are two clinical presentations of the same condition. Histologically, there is an accumulation of lipofuscin-like material in the retinal pigment epithelium cells due to a mutation in the ABCA4 gene. This mutation is transmitted when both parents carry the defective gene.
Stargardt disease causes blurred and unclear vision, making it difficult to recognize faces and shapes, and to read both near and far. Over time, it may also lead to confusion between similar color shades (e.g., black and navy blue) and difficulty adapting to low light. Although it does not cause total blindness, affected individuals can lose visual acuity to the point of legal blindness.
Currently, there is no treatment for this condition, but research in this field remains very active. There are centers in Europe and the United States participating in various clinical trials aimed at finding an effective therapy for Stargardt patients. These include pharmacological approaches as well as gene and stem cell therapies.
The main pharmacological treatments are divided into two major groups: visual cycle modulators and complement inhibitors. The Institut de la Màcula, in close collaboration with the Barcelona Macula Foundation (BMF), is the only center in Spain participating in a clinical trial aimed at testing the efficacy and safety of Zimura® (IVERIC bio, phase 2b multicenter study: OPH2005) in patients with Stargardt disease.
Retinitis pigmentosa (RP) is a hereditary and degenerative eye disease that causes a severe reduction in visual capacity and often leads to blindness. Affecting 1 in 4,000 people, more than 15,000 individuals are diagnosed with RP in Spain, and it is estimated that 60,000 people are carriers of the defective genes responsible for the condition, making them potential transmitters. The disease usually appears in young individuals, with early symptoms including difficulty adapting to darkness and progressive loss of peripheral vision.
Retinitis pigmentosa describes a group of inherited retinal diseases characterized by the progressive loss of photoreceptors (apoptosis), primarily rod cells, and of the retinal pigment epithelium, due to mutations in specific proteins and enzymes. A hereditary component is present in half of all RP cases, and prognosis as well as disease progression may be influenced by the pattern of inheritance.
Currently, there is no effective treatment to combat RP. Identifying the gene responsible for the disease is a fundamental step in the clinical management of RP patients, particularly in view of gene therapy. It allows for not only confirmation of the diagnosis, but also for establishing a genotype/phenotype correlation, offering a more accurate prognosis and potentially determining the most suitable future treatment option.
Today, researchers at the Barcelona Macula Foundation have launched the DRUG4SIGHT project, funded by La Caixa in collaboration with IBEC (Institute for Bioengineering of Catalonia) and other national entities, to develop new drugs for patients with retinal degenerations. This project takes a completely different approach from other therapeutic strategies. It aims to discover and characterize drugs that can stimulate proteins still present in the degenerated retina, enabling remaining non-degenerated cells to act as light-sensitive photoreceptors. Preliminary results are promising, but they must be confirmed in animal models with a visual system more similar to that of humans.
Choroideremia
Choroideremia (OMIM 300390) is a recessive disease caused by mutations in the CHM gene, located on chromosome Xq21.2. The mutation leads to a deficiency of a protein known as Rab escort protein 1 (REP1), which is believed to be involved in intracellular trafficking and/or rod cell function. It typically affects males (1:50,000), while females are carriers.
The disease causes the loss of the choriocapillaris (the innermost layer of the choroid), the retinal pigment epithelium, and photoreceptors (primarily rods). It begins in the mid-peripheral retina and progresses centripetally toward the macula, with characteristically well-defined borders between affected and healthy retina. Symptoms usually begin in youth. Patients experience peripheral vision loss and worsening night vision, which eventually progress to central vision loss, in a pattern similar to that seen in retinitis pigmentosa.
Although there is no treatment available, choroideremia is one of the hereditary diseases with the highest number of registered interventional studies (15 listed on www.clinicaltrials.gov, accessed December 3, 2019). Since it is a monogenic disease caused by a mutation in a single gene, gene therapy—where the defective gene is replaced with a functioning one—is being tested with promising results. Trials are ongoing in many countries, including a phase III clinical trial in the U.S. and Europe (STAR study).
Leber Congenital Amaurosis
Leber congenital amaurosis (LCA) is typically an autosomal recessive disease present at birth or shortly thereafter. Many different gene mutations have been identified as responsible for its development. It is estimated to have a prevalence of 1:40,000 and may be associated with other systemic conditions (such as developmental delay, kidney dysfunction, etc.).
LCA is characterized by retinal degeneration, in which an initially normal-looking fundus gradually progresses to generalized pigmentary changes, vascular attenuation, and retinal and optic nerve atrophy. Vision is severely impaired, and infants may show signs such as nystagmus (involuntary eye movements), photophobia (abnormal sensitivity to light), or strabismus (misalignment of the eyes).
In most cases, there is no available treatment, although some associated conditions (e.g., high hyperopia, cataracts) may be addressed. However, about 10% of LCA cases are due to mutations in the RPE65 gene (LCA type 2, OMIM 180069), and for these patients, Luxturna® (voretigene neparvovec, by Spark Therapeutics) may be used. This gene therapy replaces the defective gene with a functional one and has shown good visual outcomes. In January 2018, it was approved by the U.S. FDA (Food and Drug Administration), and in April 2019, by the Spanish Agency for Medicines and Medical Devices (AEMPS). This marked a milestone as the first gene therapy in ophthalmology to receive approval from a regulatory agency.
X-Linked Retinoschisis
X-linked retinoschisis (OMIM 312700) is a bilateral eye disorder that appears during childhood and is caused by mutations in the RS1 gene located on chromosome Xp22.13. As the name indicates, it is inherited in an X-linked pattern. It affects only males (1/5,000–1/25,000), while females are carriers and have a 50% chance of passing the mutation to their children.
The mutated RS1 gene in X-linked retinoschisis encodes retinoschisin, an adhesive protein involved in the structural and functional integrity of the retina. The fundus is characterized by cysts in the foveal area and radial striations. Affected individuals show reduced central vision and difficulties with reading. Vision loss tends to stabilize during youth, with a secondary decline occurring around ages 50–60.
Although there is no approved treatment for this condition, two clinical trials are currently active (according to www.clinicaltrials.gov, consulted December 3, 2019) to evaluate the safety, tolerability, and efficacy of a gene therapy treatment.
Some complications secondary to this disease include retinal detachment (5–22%) and vitreous hemorrhage (4–40%). Therefore, even though no treatment is currently available, regular check-ups are recommended to prevent or promptly treat any complications.
Gyrate Atrophy
Gyrate atrophy (OMIM 258870) is a rare disease caused by mutations in the gene that encodes ornithine aminotransferase (OAT), located at 10q26. The deficient activity of this enzyme leads to hyperornithinemia, although the exact mechanism by which this results in chorioretinal atrophy remains unknown. It is inherited in an autosomal recessive pattern and its prevalence is unknown (estimated at 1/50,000). The age of onset varies (from 1 month to 44 years).
Early symptoms include night blindness and reduced peripheral vision caused by multiple circular areas of chorioretinal atrophy in the retinal periphery. Over time, these atrophic areas enlarge and converge toward the macula, eventually leading to central vision loss. Patients may also present with associated myopia with significant astigmatism, early-onset posterior subcapsular cataract, and cystoid macular edema.
Several studies have reported that a diet restricted in arginine (a precursor of ornithine) or a low-protein diet can reduce serum ornithine levels and thereby slow the progression of chorioretinal atrophy and vision loss. For this reason, it is especially important to consider this disease, as dietary intervention may help delay its progression. Although some gene therapy trials have been conducted, none are currently active (according to www.clinicaltrials.gov, consulted December 3, 2019).
Fundus Albipunctatus
Fundus albipunctatus (OMIM: 136880) is one of the hereditary retinal dystrophies that typically appears in childhood. This condition is inherited and may follow an autosomal dominant or recessive pattern; its prevalence is unknown. The dominant form is caused by a mutation in the PRPH2 gene, while the recessive form is associated with a mutation in the RDH5 gene.
The RDH5 gene is involved in the visual cycle. It provides instructions for the enzyme 11-cis retinol dehydrogenase 5, which plays an important role in converting light signals into electrical signals. Mutations in this gene lead to a deficiency of the enzyme, disrupting the visual cycle and, consequently, vision—especially in low-light conditions.
Ocularly, fundus albipunctatus is characterized by numerous small, round, yellow-white retinal lesions, mainly located in the mid-peripheral retina, with no macular involvement.
Patients affected by this disease experience non-progressive night blindness (with prolonged adaptation times from light to darkness), making it important to distinguish it from other progressive conditions with similar symptoms, such as retinitis pigmentosa. Vision under normal lighting conditions is preserved.
Currently, a study is being conducted in the United States to register patients with hereditary degenerative retinal diseases in a database. The creation of such databases will allow for a better understanding of the disease and its natural history, as well as provide data on its prevalence.
Best Vitelliform Macular Dystrophy
Best vitelliform macular dystrophy (BVMD) (OMIM 153700) is a condition that begins in childhood or adolescence. It has a prevalence of 1 to 9 cases per 100,000 people, affects males more than females (3:1), and follows an autosomal dominant inheritance pattern.
In most cases, BVMD is caused by a mutation in the BEST1 gene (located on chromosome 11q12). This gene encodes bestrophin-1, a chloride channel expressed in the retinal pigment epithelium. Mutations in this protein cause the abnormal accumulation of lipofuscin due to disrupted ion exchange.
BVMD is characterized by a yellowish lesion in the macula that resembles an egg yolk, due to the abnormal build-up of lipofuscin between the photoreceptors and the retinal pigment epithelium. In more advanced stages, atrophy of the retinal pigment epithelium occurs. Patients typically experience a decrease in central visual acuity, metamorphopsia, protanopia, and a reduced Arden ratio on electrooculography. Peripheral vision and dark adaptation remain normal.
Over time, BVMD may progress to geographic atrophy, and one possible complication is the development of a choroidal subfoveal neovascular membrane, although this is rare in children.
Currently, treatment focuses on managing complications associated with the disease, such as the use of anti-VEGF agents if a subfoveal choroidal neovascular membrane develops. Recent studies have helped identify different autofluorescence patterns in this condition. Long-term monitoring is important, as changes in clinical autofluorescence can improve our understanding and prognosis of the disease according to its pattern. Additionally, studies involving gene and stem cell therapy are ongoing in the United States.
Usher Syndrome
Usher syndrome (US) is an autosomal recessive hereditary disease characterized by the combination of sensorineural hearing loss (usually congenital) with retinitis pigmentosa (RP) and progressive vision loss. The onset typically occurs during childhood and its prevalence is estimated at 1–9 per 100,000 people (in Europe, 3–4 per 100,000).
Three types of US have been identified based on differences in auditory and vestibular function (retinitis pigmentosa is common to all three types): (a) type 1, where hearing loss is congenital, profound, and vestibular function is absent; retinitis pigmentosa does not appear at birth and the mutations involve five genes (MYO7A, USH1C, CDH23, PCDH15, USH1G) and one locus (USH1E); (b) type 2, with less severe congenital hearing loss and preserved vestibular function, involving mutations in the USH2A, GPR98, and DFNB31 genes and possibly a locus on chromosome 15q; and (c) type 3, with later onset of both hearing loss and RP, associated with mutations in the CLRN1 gene.
Despite extensive research on this syndrome, no treatment currently exists. Investigations are focusing on the development of gene therapies via subretinal injections, intraocular implants of neuroprotective agents, or so-called “bionic eyes.” A multidisciplinary team approach is essential, including ENT specialists, speech therapists, and psychologists. Cochlear implants may help preserve some hearing capacity.
Cone Dystrophy
Cone dystrophy (OMIM 602093) refers to a group of hereditary retinal diseases with either autosomal recessive or dominant inheritance patterns. It typically manifests during childhood or early adulthood, and its prevalence is unknown, although in the case of cone-rod dystrophy, it is estimated at around 1 in 40,000 people.
The hallmark symptoms are progressive loss of visual acuity, color vision impairment, and photophobia (abnormal sensitivity to light). In electroretinography, only the cones are affected, while rod function is preserved. In some advanced cases, rods may also become affected, in which case the condition is referred to as cone-rod dystrophy. These cases show rod involvement in electroretinographic testing. Ophthalmoscopically, these hereditary retinal dystrophies are characterized by pigment deposits in the macular region, sometimes forming a “bull’s-eye” appearance.
Both cone dystrophy and cone-rod dystrophy have been associated with mutations in the GUCA1A gene and chromosomal regions 6q, 17p, and 19q. Other diseases that selectively affect cones include achromatopsia and blue cone monochromacy. Mild color vision deficiencies are distinct from cone dystrophy.
Currently, no treatment exists to halt the progression of this condition or to restore vision, but there are strategies aimed at helping patients cope with the social and psychological impact of visual loss. Low vision aids (magnification systems, filters) can improve quality of life for affected individuals.
Bibliography
Yannuzi, L. A. (2016) The Retina Atlas. Elsevier
Agarwal, A (2011) Glass’ Atlas of Macular Diseases. Elsevier
Online Mendelian Inheritance in Man (2019). Available at http://www.omim.org.
Clinical Trials (2019). Available at http://www.clinicaltrials.org
The portal on rare diseases and orphan drugs (2019). Available at http://www.orpha.net
Authors: Clara Abadías, Marc Biarnés, Miriam Garcia, and Cristina Romero