TRIALS
GALE: evaluation of the long-term safety and efficacy of pegcetacoplan in patients with GA secondary to AMD
STATUS:
DESCRIPTION
Objectives
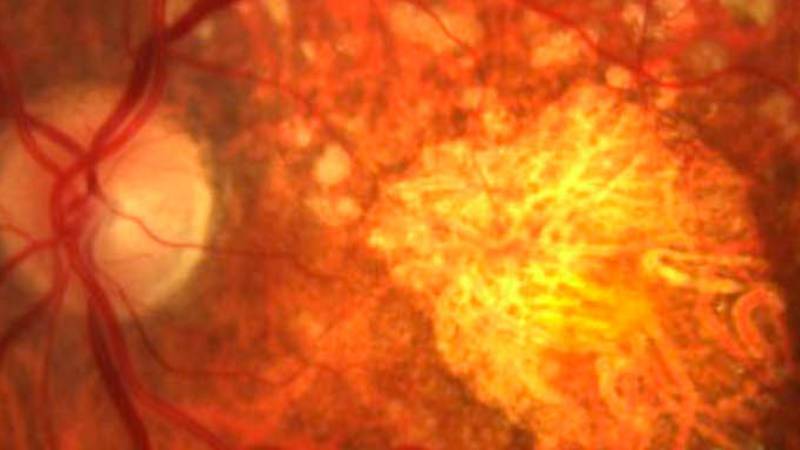
The primary objective of this study is to evaluate the long-term safety of intravitreal pegcetacoplan, as well as lesion changes measured by autofluorescence.
Drug
Biochemical, genetic, and clinical evidence in humans indicates that the complement system plays a role in the etiology of AMD.
Complement protein C3 (which has a pro-inflammatory action), the membrane attack complex, and complement factor H are present in drusen and basal laminar deposits in patients with AMD.
Pegcetacoplan is an intravitreal drug that blocks the action of C3. Its main effect is anti-inflammatory.
Population
This study only includes patients who have participated in the APL2-103 study or have completed month 24 of treatment in the APL2-303 (DERBY) or APL2-304 (OAKS) studies.
Duration
Associated Pathologies
No results found.
If you found this interesting, feel free to share it here:

